These instruments are used when there is a necessity of liquid control at one specific point or between maximum and minimum values. Their principle is based on the measurements of electrolytic conductivity of liquids.
Two electrodes 1 and 2 are immersed in liquid 3, which fills a vessel 4 in Fig. These electrodes through electric cables 5 are connected with an electric or electronic relay 6. Electrodes should be insulated from the vessel. Each of the electrodes forms an electrical circuit with the vessel through liquid.
Therefore, the material of the vessel should be conductive. When liquid forms the circuit with the electrode 1, the electric relay starts to operate, and a signal is sent to a secondary device which detects that the lower limit of the level equal to hmin has been reached. When liquid is in contact with the electrode 2, this indicates that the upper limit of the level hmax has been reached.
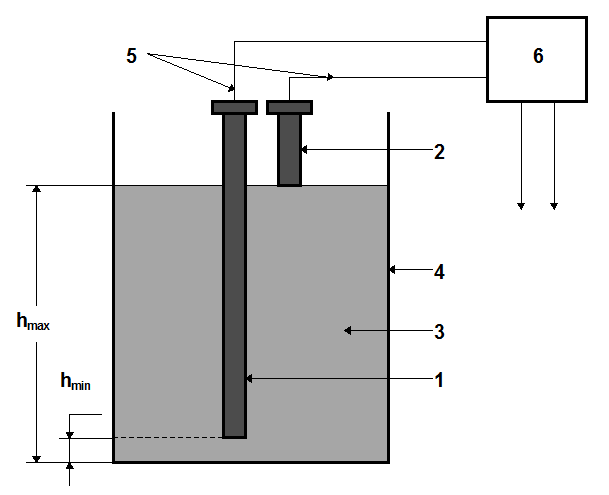
These devices may be used for an interface-level control, where one liquid is conductive, whereas the second liquid is dielectric. The advantage of conductive-type level meters is that they can be used in vessels under atmospheric or manometric (or vacuumetric) pressures. When employing electric relays, the best results may be achieved for the most of aqueous solutions of electrolytes with electrolytic resistivities lower that 20000 Ohm*cm. If one deals with liquids with low electrolytic conductivity (water, alcohol), the sensitivity of an electric circuit becomes lower, so electronically operated relays are used to increase the sensitivity of the device.
Article Source:: Dr. Alexander Badalyan, University of South Australia