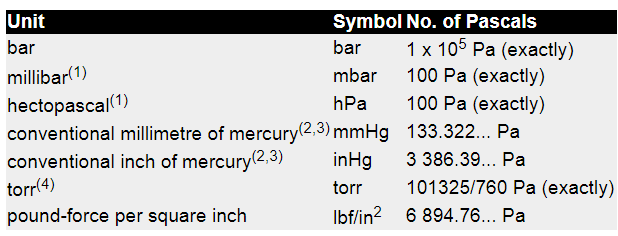
The SI unit of pressure is the pascal, symbol Pa, the special name given to a pressure of one newton per square metre (N/m2). The relationships between the pascal and some other pressure units are shown in the table but note that not all are, or can be, expressed exactly. Note also that the term standard atmosphere is not a pressure unit(5).
Notes:
(1) Following the 8th Congress of the World Meteorological Organization, from 1 January 1986 the term hectopascal (hPa) is preferred to the numerically identical millibar (mbar) for meteorological purposes. This choice was made, despite the fact that hecto (x 100) is not a preferred multiple in the SI system, to avoid having to change the numerical values on barometer scales.
(2) The conventional millimetre of mercury (mmHg) and the conventional inch of mercury (inHg) are defined in terms of the pressure generated by a mercury column of unit length and of assigned density 13595.1 kg/m3 at 0 °C under standard gravity of 9.80665 m/s2. (See note 3 and BS 2520 : 1983 Barometer conventions, their application and use).
(3) The so-called ‘manometric’ pressure unit definitions such as millimetres of mercury and inches of mercury depend on an assumed liquid density and acceleration due to gravity, assumptions which inherently limit knowledge of their relationship with the pascal. In order to encourage the demise of non-SI units, whose definitions are becoming inadequate for the most precise measurement of pressure, there is international effort to exclude them from conversion tables or, in the meantime, restrict the precision of newly published conversion factors. It is strongly recommended that, wherever possible, all new applications of pressure measurement use the pascal, with multiples or sub-multiples as appropriate to the magnitude of the pressure values.
(4) The torr is defined as exactly 101325/760 Pa - the ‘760’ coming from the original and arbitrary definition of standard atmosphere. Its value differs from the conventional millimetre of mercury by about 1 part in 7 million. (See BS 2520 : 1983 Barometer conventions, their application and use).
(5) The definition of standard atmosphere (atm) is 101 325.0 Pa exactly. It is still occasionally used in defining a reference environment - eg for specifying gas density - but it is not a pressure unit and should not be used to express pressure values. The large number of significant figures used to define it and its far-from-round-number appearance sometimes leads to the erroneous assumption that it is the measured average pressure on the surface of the earth. It is not and such a measurement could not realistically be made. (Consider how many locations would be used and over what timescale? Changing either of these parameters would change the result.)