Zirconia oxygen analyzers determine oxygen concentration using the conductivity of a zirconia ceramic cell. Zirconia ceramic cells only allow oxygen ions to pass through at high temperatures.
With reference gas on one side and sample gas on the other, oxygen ions move from the side with the highest concentration of oxygen to that with the lowest concentration.
The movement of ions generates an EMF (Electro Motive Force) which can be measured to determine the oxygen content.
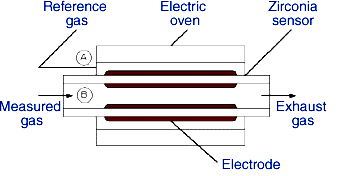
In Fig., a gas with fixed oxygen concentration (normally air) is placed on the A side as the reference gas (PR), while the gas whose oxygen concentration is to be measured (PM) is placed on the B side. When this is done, ion conduction occurs from the side with higher oxygen concentration to the side with lower oxygen concentration. This generates an voltage (emf) which is proportional to measured oxygen gas concentration.
Source : toray-eng