Pressure Control in Two Phase Systems
An example of a process which contains both a vapour and a liquid is distillation. We generally wish to regulate the pressure in the column, which contains mainly vapour. This could be done by placing a valve in the vapour line leaving the column, exactly as we did with the simple tank.
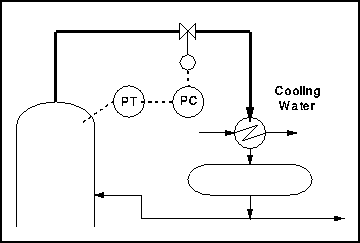
There are several disadvantages to this system. One is that the control valve is on a vapour line. These are generally much bigger than liquid lines and hence require a much bigger valve i.e. of a much increased cost.
However, we remember that in a two phase system temperature and pressure are not independent . We can thus change the pressure of a vapour which is in equillibrium with a liquid by changing the temperature of the system. Raising the temperature raises the vapour pressure of the liquid which must equal the equillibrium pressure of the system.
Hence we can manipulate the temperature in the condenser by means of a small valve on the cooling water line, thus changing the pressure in both condenser and column.
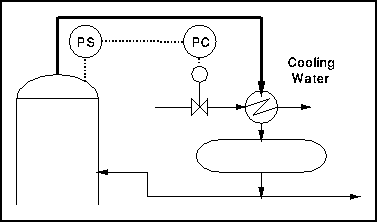
There are a large number of different ways of manipulating pressure in two phase systems. These are discussed in a later section under Control of Distillation Process.